Takeaways from the Empowering Women in Organic Chemistry Europe (EWOC) Conference, 2025
By May Merino, sub-editor
This year’s Empowering Women in Organic Chemistry (EWOC) Europe Conference was hosted at the Novartis site in Basel, Switzerland. With the aim of promoting, supporting, and empowering gender minorities in the field of organic chemistry, attendees included organic chemists across the spectrum of academia and industry. The conference is both a scientific and advocacy-focused event, combining comprehensive chemistry presentations with meaningful conversations about equity and representation in science. Active allyship is a key requirement for understanding, learning from and progressing beyond current gender issues, and it was encouraging to see a higher number of male attendees compared to the previous conference held in Birmingham.
Leading by example: Dr Rebecca Ruck, MSD
There were many amazing talks but one that stood out in particular was from Dr Rebecca Ruck, MSD, who is also a founding member of the EWOC movement. Dr Ruck opened by sharing her perspective on intersectionality as both a woman in chemistry and someone living with Crohn’s Disease. She described her experience as a “super power”, due to the empathy she felt for others also facing chronic illness. In the spirit of allyship and supporting others, she was careful to draw attention to several female colleagues who played key roles in the chemistry she later discussed.
Ruzasvir: Mechanistic understanding for efficient catalysis
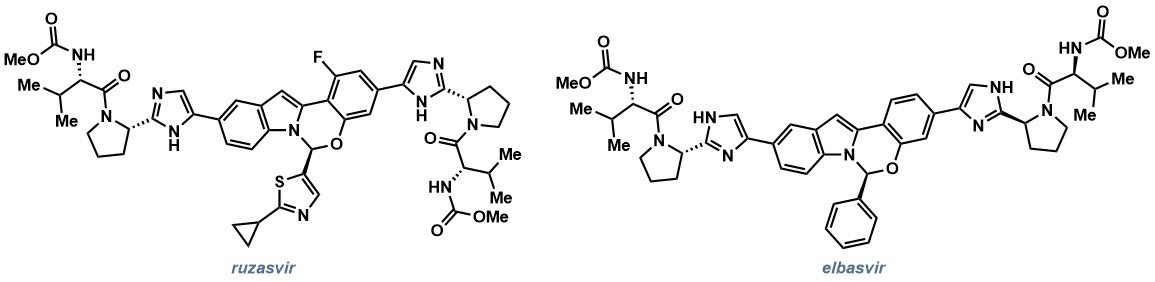
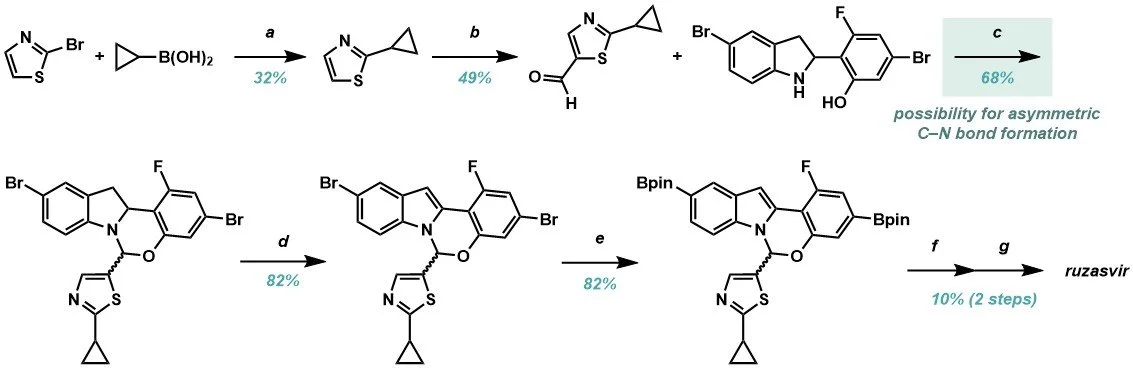
One focus of the talk was the development of a key catalytic step for the synthesis of ruzasvir (Figure 1a), a compound currently being investigated for the treatment of hepatitis C. In the discovery route (Scheme 1) the hemiaminal was formed as a racemic mixture, with chiral separation employed in the final step to isolate the desired enantiomer. However, the Process Team identified an opportunity to form the key C–N bond asymmetrically, while working on a related compound, elbasvir (Figure 1b), which would enable more efficient synthesis of ruzasvir, as well as potentially reducing waste streams and environmental impact.
Figure 1. NS5A inhibitors for the treatment of Hepatitis C. (a) Ruzasvir; (b) Elbasvir
Scheme 1. Discovery route to Ruzasvir. (a) Suzuki-Miyaura coupling; (b) aromatic formylation; (c) acid-catalysed cyclisation; (d) aromatisation to indole; (e) Miyaura borylation; (f) Suzuki-Miyaura coupling; (g) chiral separation to deliver final product.
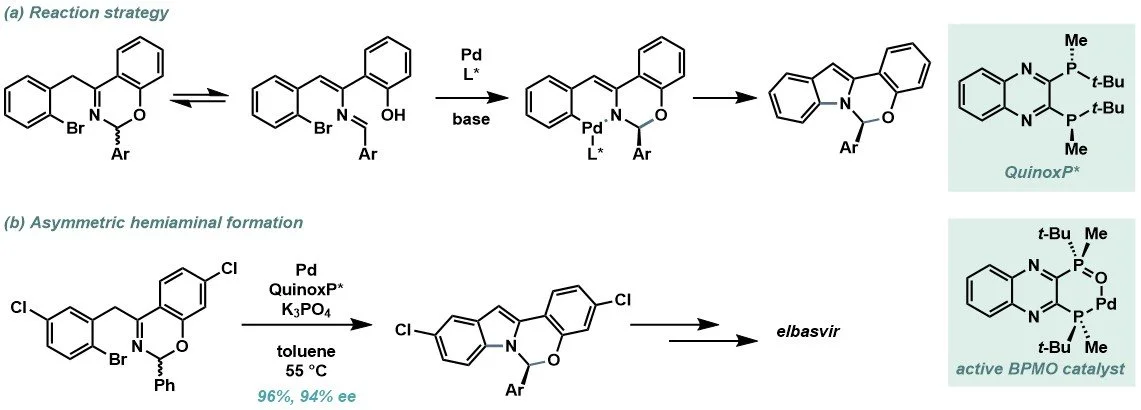
During route development of elbasvir, it was envisaged that the use of chiral phosphine ligands in palladium-catalysis would enable stereochemical control in the Buchwald–Hartwig amination (Scheme 2a). QuinoxP*, a chiral phosphine ligand, was the most effective additive, furnishing the desired hemiaminal in an impressive 96% yield and 94% enantiomeric excess (Scheme 2b). Under the reaction conditions, mono-oxidation occurs where the hemi-lability of the resulting bisphosphine monoxide (BPMO) is essential for oxidative addition and asymmetric catalysis.
Scheme 2. (a) Use of chiral phosphine ligands for stereoselective C–N bond formation. (b) Asymmetric hemiaminal formation step for the synthesis of elbasvir. (c) QuinoxP* and active Pd(0)–BMPO catalyst.
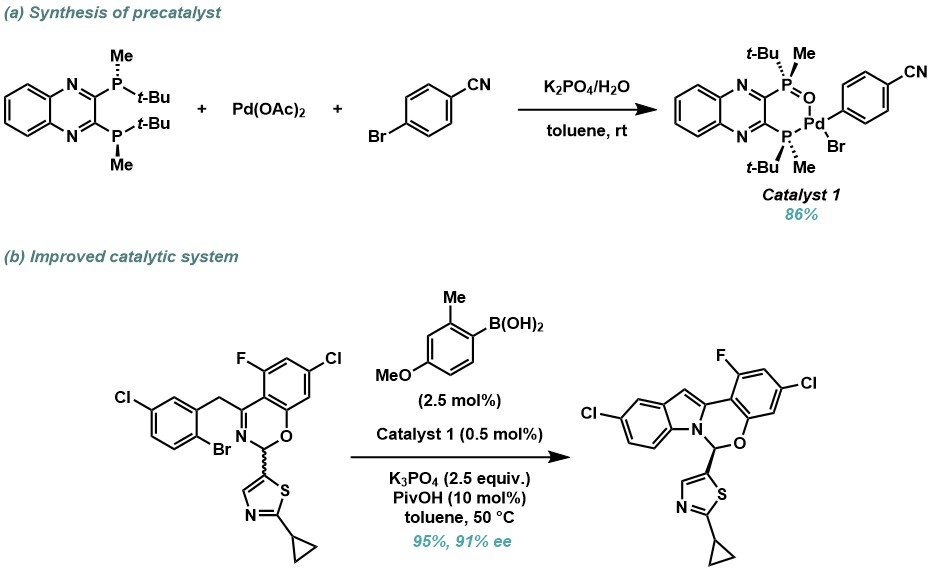
This methodology translated well to ruzasvir but required increased catalyst loading from 1 mol% to 5 mol%, which was considered too costly for large scale manufacturing. Detailed mechanistic investigation into the C–N coupling found that formation of the active Pd(0)–BMPO catalyst was inefficient under the current conditions. Subsequently, a new class of air-stable BPMO–Pd(II) pre-catalysts were designed, which were readily prepared from oxidative addition of aryl halides with palladium acetate and the QuinoxP* ligand (Scheme 3a). These pre-catalysts can then be activated in situ through Suzuki–Miyaura reaction with an aryl boronic acid, with the new system proving highly effective, enabling a ten-fold reduction in catalyst loading from 5 to 0.5 mol% while maintaining excellent yield and enantioselectivity (Scheme 3b).
Scheme 3. (a) Synthesis of BPMO–Pd(II) precatalysts. (b) New catalyst system for the asymmetric hemiaminal formation.
Reflections from EWOC25
This was just one talk from the many insightful and inspiring presentations during the EWOC conference. Overall, exceptional science instigated by, and led by, women in the field was showcased, which provided an energising and welcoming environment in which to network with chemists from across industry and academia. It also provided a platform to discuss challenges faced by women and gender minorities within the organic chemistry community. Topics such as imposter syndrome, family responsibilities and navigating career progression were dissected and discussed, with attendees sharing their own experiences. While some of the statistics shared, especially around the leaky pipeline, made uncomfortable listening, ultimately the importance of events like EWOC and the establishment of a community of scientists with shared-experiences was highlighted as a positive in supporting others entering the field of organic chemistry.